Abstract
Introduction: Autologous hematopoietic stem cell transplantation (autoHCT) is considered standard of care for patients with multiple myeloma (MM). However, most patients eventually relapse, perhaps due to the presence of clonal plasma cells (CPC) in the autograft. The significance of CPC in the autograft has not been clearly established. MM patients with high-risk chromosomal abnormalities (HRMM) by florescent in situ hybridization (FISH) have worse outcomes compared to patients with standard-risk disease. We conducted a single center retrospective analysis to evaluate the impact of CPC in the autograft on the outcomes of patients with HRMM undergoing an autoHCT at our institution.
Methods: Eligible patients had HRMM, received an autoHCT between 2008 and 2018 at our institution, and had 8-color next-generation flow cytometry (NGF) performed on the collected apheresis product for CPC. HRMM was defined as having del17p, t(4;14), t(14;16) and 1q21 gain or amplification by FISH. Patients were divided into two groups: CPC+ or CPC- in the autograft by NGF. Since not all collected autograft products are infused at autoHCT, and some bags may be CPC+ or CPC- for the same patient, we also evaluated the impact of infusion of CPC+ autograft products on patient outcomes. Minimal residual disease (MRD) status on the bone marrow biopsy was evaluated using 8-color next generation flow cytometry with sensitivity of 1/10-5 cells.
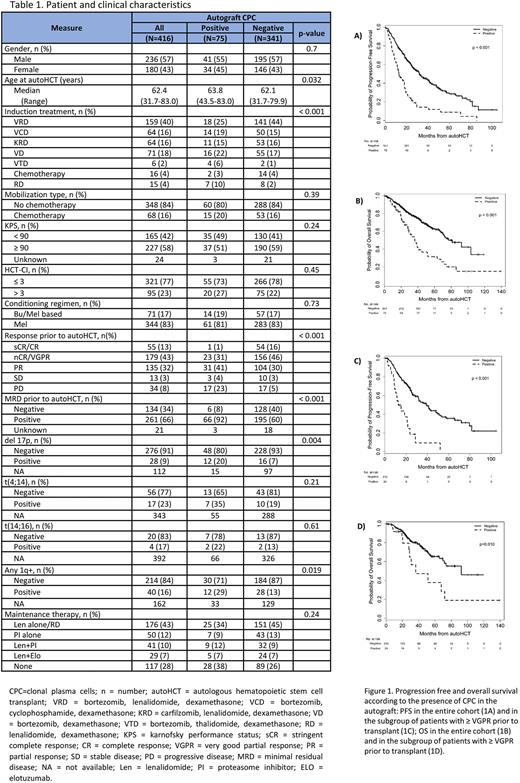
Results: We analyzed the autograft products of 416 patients: 75 CPC+ (18%) and 341 CPC- (82%). 57% patients were male, and median age at autoHCT was 62 (range 32-83) years. Fewer patients in the CPC+ group received the VRD regimen as induction prior to transplant compared to the CPC- group (25% vs. 44%, p=0.003) and fewer achieved ≥VGPR after induction compared to the CPC- group (32% vs. 62%, p<0.001). Patient and clinical characteristics are presented in Table 1.
Median follow up for the whole cohort was 35.7 (range 0.3-139.5) months. 100-day and best post-autoHCT CR rates in the CPC+ and CPC- groups were 8% vs. 19% (p<0.001), and 33% vs. 54% (p<0.001), respectively. The CPC+ group was also less likely to achieve MRD-negative CR post-transplant (11% vs. 42%; p<0.001). Median PFS in the CPC+ vs. CPC- group was 12.8 vs. 32.1 months (p<0.001), and median OS was 36.4 vs. 81.2 months (p<0.001).
The degree of autograft CPC involvement was inversely associated with post-transplant day 100 response: mean maximal autograft CPC level was 0.02% (SD 0.02) for patients who achieved ≥VGPR vs. 1.04% (SD 2.59) for those who achieved ≤PR at day 100 post-transplant. Similarly, degree of autograft CPC level was inversely associated with best post-transplant response: mean maximal autograft CPC level was 0.06% (SD 0.16) for patients who achieved ≥VGPR vs. vs. 1.55% (SD 3.17) for those who achieved ≤PR.
In patients achieving ≥VGPR prior to autoHCT, those with CPC+ autograft had inferior PFS (hazard ratio (HR) [95% CI]: 3.38 [2.05-5.58], p<0.001; Figure 1C) and OS (HR 2.29 [1.20-4.40], p=0.013; Figure 1D) compared to the CPC- group. Also in patients who achieved MRD- negative CR or VGPR prior to autoHCT, those with CPC+ autograft had inferior PFS (HR 4.35 [1.87-10.16], p<0.001) and OS (HR 6.14 [2.10-17.96], p<0.001) compared to the CPC- group. In a multivariable analysis (MVA), the degree of CPC positivity in the autograft was independently predictive of worse PFS (HR 1.50 [1.12-1.99]; p=0.006) and OS (HR 1.35 [1.08-1.67]; p=0.007). The MVA also showed that infusion of CPC+ autograft products was predictive of worse PFS (HR 2.78 [1.85-4.15]; p<0.001) with a trend towards worse OS (HR 1.66 [0.99-2.78]; p=0.054).
Conclusions: Our study shows a major impact of CPC in the autograft on post-autoHCT outcomes in HRMM. Both the presence and degree of CPC in the autograft were highly predictive of inferior PFS and OS, even in patients who had achieved ≥VGPR and MRD-negative CR/VGPR prior to autoHCT. Novel strategies for ex-vivo purging of CPC could improve patient outcomes.
Disclosures
Srour:Orca Bio: Research Funding. Nieto:Secura Bio: Research Funding; Affimed: Other: Scientific advisory Board, Research Funding; Astra Zeneca: Research Funding. Lee:Legend Biotech: Consultancy; Karyopharm: Consultancy; Pfizer: Consultancy; Immunitas Therapeutics: Consultancy; Janssen: Research Funding; Regeneron: Research Funding; Takeda Pharmaceuticals: Consultancy, Research Funding; Sanofi: Consultancy; GSK: Consultancy, Research Funding; Monte Rosa Therapeutics: Consultancy; Genentech: Consultancy; Amgen: Research Funding; Oncopetides: Consultancy. Patel:Janssen, Celgene/BMS, Caribou Sciences, Arcellx, Cellectis, Merck, Pfizer, Karyopharm, Oncopeptides: Consultancy. Kebriaei:Ziopharm: Research Funding; Amgen: Research Funding; Pfizer: Consultancy; Kite: Consultancy; Jazz: Consultancy. Thomas:Bristol Myers Squibb: Research Funding; Genentech: Research Funding; X4 Pharma: Research Funding; Janssen Pharma: Research Funding; Cellectar Pharma: Research Funding; Ascentage Pharma: Research Funding. Orlowski:Abbvie, BioTheryX, Inc., Bristol-Myers Squibb, Janssen Biotech, Karyopharm Therapeutics, Inc., Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda Pharmaceutic: Honoraria, Membership on an entity's Board of Directors or advisory committees; CARsgen Therapeutics, Celgene/Bristol Myers Squibb, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding; Asylia Therapeutics, Inc.: Current equity holder in private company; Asylia Therapeutics, Inc., BioTheryX, Inc., Heidelberg Pharma, Inc.: Research Funding. Rezvani:Bayer: Other: Participates on the Scientific Advisory Board ; Navan Technologies: Other: Participates on the Scientific Advisory Board ; GSK: Other: Participates on the Scientific Advisory Board ; Virogin Biotech: Other: Participates on the Scientific Advisory Board ; AvengeBio: Other: Participates on the Scientific Advisory Board ; GemoAb: Other: Participates on the Scientific Advisory Board ; Takeda: Patents & Royalties, Research Funding; Affimed: Research Funding; Caribou Biosciences: Other: Participates on the Scientific Advisory Board . Shpall:axio: Consultancy; Takeda: Patents & Royalties; Navan: Consultancy; adaptimmune: Consultancy; Fibroblasts and FibroBiologics: Consultancy; Bayer: Honoraria; NY blood center: Consultancy; Affimed: Other: License agreement. Champlin:Bluebird: Other: Data Safety Monitoring Board; General Oncology: Other: Data Safety Monitoring Board; Actinium: Consultancy; Kadmon: Consultancy; Omeros: Consultancy; Cell Source Inc.: Research Funding; Johnson &Johnson: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal